Visiting scholar in the D'Esposito Lab at the University of California, Berkeley, researching the relationship between regional function and brain network architecture supporting complex executive function
investigating the regions and networks of complex behavior
About Me
I’m a visiting scholar in Dr. Mark D’Esposito’s lab at the University of California, Berkeley, having completed my postdoctoral fellowship in December 2022. I am trained as a cognitive neuroscientist and experimental psychologist. My research aims to understand how the different scales of functional organization observed in the brain – for example, regional and network-level – work together to support complex behavior.
I completed my undergraduate degree in Biomedical Engineering at the Georgia Institute of Technology in 2011. As an undergrad, I worked as a research assistant with Dr. Michelle LaPlaca developing in vitro models of secondary pathways of cell damage response. This led me to an interest in cognitive function and its underlying neural mechanisms.
In Fall 2011, I started graduate school with Dr. Eric Schumacher in the School of Psychology at the Georgia Institute of Technology. My research focused on how brain regions controlled preparation and execution of tasks, especially in the lateral frontal cortex. This research combined experimental psychology and functional magnetic resonance imaging methods in order to link behavior with brain activity in real time.
To further explore the interactions between functional regions, in my postdoctoral research I turned toward graph theory and network neuroscience, using novel methods that allow regions to be a part of multiple overlapping networks. I received NIH F32 funding in January of 2020 to pursue a set of projects exploring these overlapping networks. In the future, I hope to understand how brain networks relate to activation of individual regions in the lateral frontal cortex, and how temporary disruption of these different regions via transcranial magnetic stimulation can lead to network-level connectivity changes.
Calendar times are in UTC; if you are in Gather, check the clock on the wall if you’re not sure what the conversion is for your timezone!
Events are color-coded to the room reserved; see which rooms are which color by clicking the top right arrow.
Brainarium users can reserve rooms in the Gather space by adding their events directly to the Google calendar; message the admins to request access if you don’t have it already. Admin approval is required to reserve the full Gather space.
Brainarium is a small start-up consortium whose goal is to bring brain scientists together from all fields, locations, and walks of life.
Even without the shift to virtual work over the past year, science can be isolating and networking can be difficult. We aim to change the social dynamics of science work and shift the way we connect with each other.
Currently, we have two primary resources, a group-wide Slack workspace and an open virtual officespace on Gather. Slack gives us a rich community with many channels for different conversation topics. Gather lets us come together in a natural office setting and have real-time interactions and casual networking opportunities.
Unlike many conferences and networking events, these spaces are permanent. This means that at any time, anywhere, you can connect with your Brainarium colleagues both through asynchronously and face-to-face.
Now, we need people! Joining is completely free. Send your institution-verified email address to joinbrainarium [at] gmail [dot] com to get access. Anyone who works in brain science in any capacity and at any level of training is welcome. Join today!
Introduction
Network neuroscience analyzes the connections between regions of the brain to assess the community structure of those connections and the role of different regions in communication across those communities. The regions in these communities may be defined by one of many existing parcellation definitions, which can be highly variable in their resolution and topography. Previous research has shown that choice of parcellation can influence network measures (Messé, 2019). Here, we asked if parcellation resolution influenced the community affiliated with a given brain location, and whether this related to changes in the average hubness of the regions in each community.
Methods
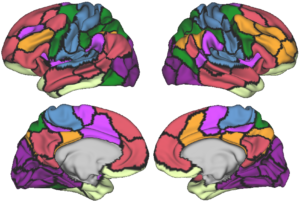
To assess how parcellation resolution influenced community assignment, we defined a set of surface-based coordinates of interest (COIs) and analyzed how many unique communities each was affiliated with across resolutions. To identify our COIs, we projected spherical volumes centered on a set of point locations (Power et al, 2011) onto a surface space and manually selected the coordinate located most centrally within the projected area. For the assignment analysis, we utilized the Global-Local parcellation set (Schaefer et al, 2018) in ten different resolutions from 100 to 1000 regions, each of which has each been mapped to a set of seven communities. We identified the parcel containing each of our COIs and its affiliated community at each resolution, then calculated each COI’s number of unique communities across resolutions. To assess how resolution influenced hubness measures of these communities, we applied a 17-community structure (Yeo et al, 2011) to the resting state data of 50 subjects from the Human Connectome Project and calculated coordinate-level metrics of participation coefficient (PC) and within-module degree (WMD). We assessed the PC and WMD for each parcel at each resolution by averaging across the values of all of the coordinates contained in that parcel. Finally, we assessed the trends in PC and WMD within each community across resolutions using repeated measures ANOVAs.
Results
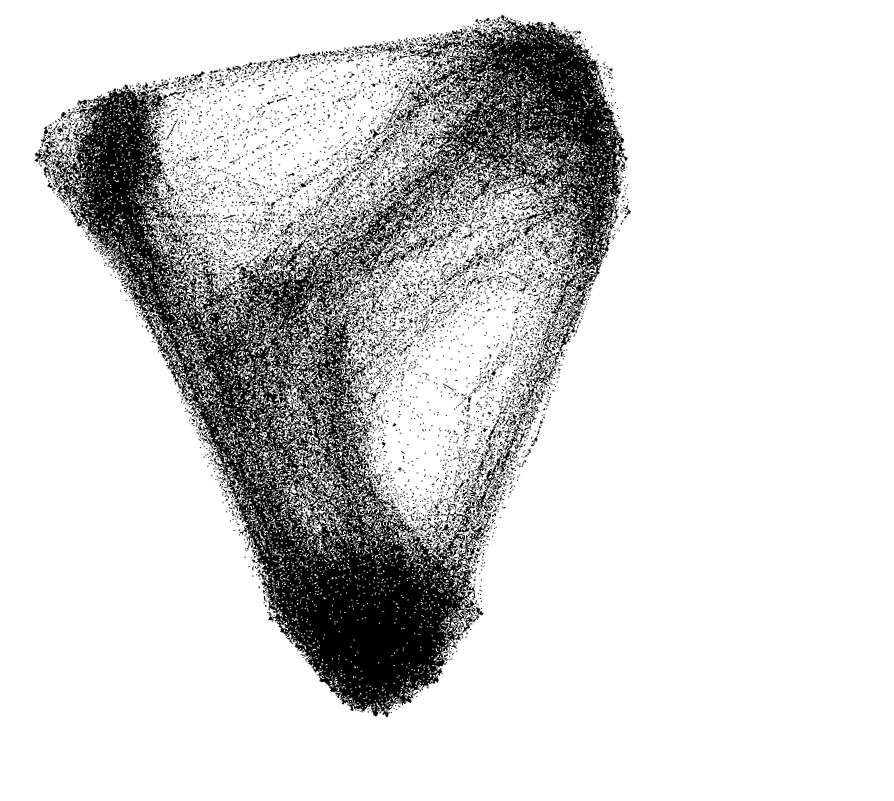
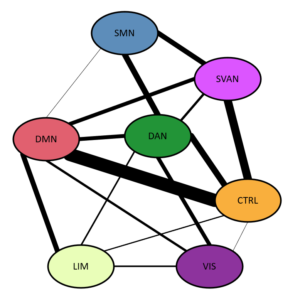
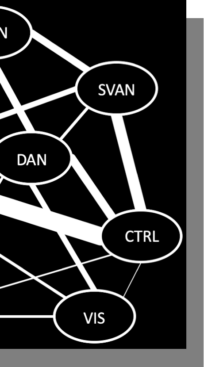
Our network assignment analysis revealed that 161 (66.8%) out of the 241 COIs were affiliated with a single community across resolutions. Of the remaining 80 regions, 64 (79.6%) were affiliated with two communities; 15 (18.8%) with three; and one with four. Mapping out the affiliations between communities revealed a dense sharing structure. The somatomotor network (SMN) was the most isolated, sharing regions almost exclusively with the dorsal (DAN) and salience/ventral attention (SVAN) networks. The visual network primarily shared regions with the DAN, while the limbic system primarily shared with the default mode network (DMN). Higher-order association networks showed a rich pattern of mutual sharing. PC and WMD showed largely consistent values across resolutions in the control, limbic, DAN, SVAN, SMN, and visual networks. The DMN showed linear relationships for both WMD (F = 7.425, p = .009, ηp2 = .134) and PC (F = 6.035, p = .018, ηp2 = .112). That is, DMN appeared more internally integrated, but more externally isolated, with increasing parcel resolution.
Conclusions
The current results show that community affiliation is critically dependent on parcellation resolution while community-level network measures are not, which suggests that each parcellation is capturing different facets of a location’s connectivity based on which connections are included in the parcel definition. They also indicate that regions shift community affiliation along a constrained set of paths that are not described by aggregate measures of hubness. Together, this suggests that studies using a single parcellation to assign regions to unique communities do not accurately or fully capture the brain’s community structure, severely limiting their capacity to interpret regional roles within the network.
References
- Messé, A. (2019). Parcellation influence on the connectivity-based structure–function relationship in the human brain. Human Brain Mapping, (November), 1–14.
- Schaefer, A., Kong, R., Gordon, E. M., Laumann, T. O., Zuo, X.-N., Holmes, A. J., … Yeo, B. T. T. (2018). Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cerebral Cortex, 28(9), 3095–3114.
- Yeo, B. T. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., … Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165.
- Power, J. D., Cohen, A. L., Nelson, S. M., Wig, G. S., Barnes, K. A., Church, J. A., … Petersen, S. E. (2011). Functional Network Organization of the Human Brain. Neuron, 72(4), 665–678.
Supplementary Materials *Updated 19 June 2020
Contents:
-
Reproduction of switching map with networks color-coded
-
Visualization of switching ROIs
-
Number of ROIs switching between specific network pairs
-
Graphs of network connectivity measures by number of networks per region
| Color | Yeo et al (2011) Network Label |
|---|---|
| Somatomotor Network | |
| Salience/Ventral Attention Network | |
| Dorsal Attention Network | |
| Default Mode Network | |
| Control Network | |
| Limbic Network | |
| Visual Network |
3. Number of ROIs that switch between specific network pairs:
| Network 1 | Network 2 | ROIs |
|---|---|---|
| Control | Default Mode | 23 |
| Control | Salience/Ventral Attention | 14 |
| Control | Dorsal Attention | 11 |
| Dorsal Attention | Somatomotor | 10 |
| Salience/Ventral Attention | Somatomotor | 9 |
| Default Mode | Limbic | 9 |
| Default Mode | Salience/Ventral Attention | 7 |
| Default Mode | Dorsal Attention | 7 |
| Dorsal Attention | Visual | 7 |
| Default Mode | Visual | 4 |
| Dorsal Attention | Salience/Ventral Attention | 4 |
| Limbic | Visual | 3 |
| Dorsal Attention | Limbic | 3 |
| Control | Limbic | 2 |
| Default Mode | Somatomotor | 1 |
| Control | Visual | 1 |
Participation Coefficient
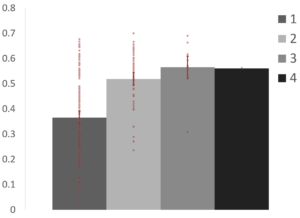
Participation coefficient significantly increased from regions in one network to those in multiple networks (p1,2 & p1,3 < .001). This suggests that regions that were assigned to different networks across resolutions have stronger connections to more than one network than those that were consistently assigned to one network.
Within-Module Degree
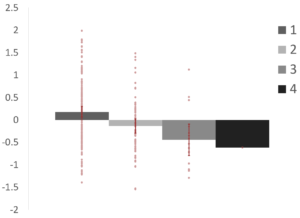
Within-module degree significantly decreased from regions in one network to those in multiple networks (p1,2 = .005; p1,3 = .012). This suggests that regions that were assigned to different networks across resolutions are more weakly connected to their assigned network than those that were consistently assigned to one network.
Mapping Systematic Changes in Community Assignment Across Parcellation Resolutions
Find more at the links below!
Abstract
Poster Reprint
Supplementary Results
Network neuroscience often relies on the use of pre-existing parcellations to define the boundaries of the brain regions that will be submitted for network assignment and subsequent analysis. We present preliminary results that indicate that the topography of network partitions varies with the number of regions included in the parcellation when the parcellation algorithm is kept constant. This may be due to implicit assumptions in network assignment methods that lose important information about the multi-network connections of many regions, especially those showing “hub-like” properties. We argue that network neuroscience will need to move toward new methods that capture this information in order to gain a full understanding of the relationship between regional processes and network communication structure.
OHBM attendees can see this work presented live on Zoom at the following dates and times (24hr format). Zoom link will be provided in the live chat window during each session.
| Date | Day | Berkeley | New York | London | Hong Kong |
|---|---|---|---|---|---|
| June 24th | Wednesday | 9:00 | 12:00 | 17:00 | 0:00 (+1d) |
| June 26th | Friday | 17:00 | 20:00 | 1:00 (+1d) | 8:00 (+1d) |
| June 30th | Tuesday | 17:00 | 20:00 | 1:00 (+1d) | 8:00 (+1d) |
| July 2nd | Thursday | 23:00 (-1d) | 2:00 | 7:00 | 11:00 |
+1d = the following calendar date; -1d = the previous calendar date
OHBM 2020
Parcellations
I’m a visiting scholar in Dr. Mark D’Esposito’s lab at the University of California, Berkeley, having completed my postdoctoral fellowship in December 2022. I am trained as a cognitive neuroscientist and experimental psychologist. My research aims to understand how the different scales of functional organization observed in the brain – for example, regional and network-level – work together to support complex behavior.
I completed my undergraduate degree in Biomedical Engineering at the Georgia Institute of Technology in 2011. As an undergrad, I worked as a research assistant with Dr. Michelle LaPlaca developing in vitro models of secondary pathways of cell damage response. This led me to an interest in cognitive function and its underlying neural mechanisms.
In Fall 2011, I started graduate school with Dr. Eric Schumacher in the School of Psychology at the Georgia Institute of Technology. My research focused on how brain regions controlled preparation and execution of tasks, especially in the lateral frontal cortex. This research combined experimental psychology and functional magnetic resonance imaging methods in order to link behavior with brain activity in real time.
To further explore the interactions between functional regions, in my postdoctoral research I turned toward graph theory and network neuroscience, using novel methods that allow regions to be a part of multiple overlapping networks. I received NIH F32 funding in January of 2020 to pursue a set of projects exploring these overlapping networks. In the future, I hope to understand how brain networks relate to activation of individual regions in the lateral frontal cortex, and how temporary disruption of these different regions via transcranial magnetic stimulation can lead to network-level connectivity changes.
Research
Publications
Evaluating the reliability, validity, and utility of overlapping networks: Implications for cognitive control
Cookson, S. L., D'Esposito, M. (2022) HBM :: Brain network definitions typically assume nonoverlap or minimal overlap, ignoring regions' connections…
Connectivity-defined subdivisions of the intraparietal sulcus respond differentially to abstraction during decision making
Newton, M., Cookson, S. L., D’Esposito, M., Kayser, A. (2022) J Neurosci :: The intraparietal sulcus (IPS) has been implicated…
Dissociating the neural correlates of planning and executing tasks with nested task sets
Cookson, S. L., Schumacher, E. H. (2022) JOCN :: Task processing (e.g., the preparation and execution of responses) and task…
Centering inclusivity in the design of online conferences – An OHBM – Open Science perspective.
Levitis, E., Gould van Praag, C., Gau, R., ... Cookson, S. L. ... Maumet, C. (2021). Gigascience :: As the…
Task sets serve as boundaries for the congruency sequence effect
Grant, L. D., Cookson, S. L., Weissman, D. H. (2020) JEPHPP :: Cognitive control processes that enable purposeful behavior are…
Task structure boundaries affect response preparation
Cookson, S. L.*, Hazeltine, E., Schumacher, E. H. (2019) Psychological Research :: Does cognitive control operate globally (across task sets)…